2
HEAT
 Although heat at first appears to have nothing to do with motion, it is
now understood that heat is the motion of molecules. The connection
between
heat and motion was provided by Benjamin Thompson (1753-1814), an
American
who sympathized with the British during the Revolutionary War and
eventually
settled in Bavaria and became Count Rumford[1]. While watching the
boring
of artillery cannons in Munich in 1798, he concluded that motion could
be transformed into heat and estimated the mechanical equivalent of
heat.*
Half a century later the experiment was refined and repeated by James
Prescott
Joule (1818-1889), the son of an English brewer. Joule's careful
quantitative
measurements became a model for modern science[2]. For our purposes, we
will include in the chapter on heat such topics as fluid mechanics,
phase
transitions, cryogenics, thermodynamics, kinetic theory and combustion.
Although heat at first appears to have nothing to do with motion, it is
now understood that heat is the motion of molecules. The connection
between
heat and motion was provided by Benjamin Thompson (1753-1814), an
American
who sympathized with the British during the Revolutionary War and
eventually
settled in Bavaria and became Count Rumford[1]. While watching the
boring
of artillery cannons in Munich in 1798, he concluded that motion could
be transformed into heat and estimated the mechanical equivalent of
heat.*
Half a century later the experiment was refined and repeated by James
Prescott
Joule (1818-1889), the son of an English brewer. Joule's careful
quantitative
measurements became a model for modern science[2]. For our purposes, we
will include in the chapter on heat such topics as fluid mechanics,
phase
transitions, cryogenics, thermodynamics, kinetic theory and combustion.
*Now defined as 4.186 joules/calorie
REFERENCES
1. S. C. Brown, Benjamin Thompson, Count Rumford, MIT Press:
Cambridge,
Massachusetts (1979).
2. H. J. Steffens, James Prescott Joule and the Concept of Energy,
Science History Publications: New York (1979).
Table 2.1
Physical Properties of Selected Gases
Molecular Freezing Boiling Density
Weight Point Point (g/liter)
Gas Formula (g/mole) (°C) (°C) (1 atm)
Argon Ar 39.95 -189.2 -185.9 1.633
Carbon dioxide CO2 44.01 -78.5a 1.799
Helium He 4.00 -272.2b -268.9 0.164
Hydrogen H2 2.02 -259.1 -252.9 0.082
Methane CH4 16.04 -182.6 -161.5 0.656
Neon Ne 20.18 -248.7 -246.1 0.825
Nitrogen N2 28.01 -209.9 -195.8 1.145
Oxygen O2 32.00 -218.4 -183.0 1.308
Sulfur hexafluoride SF6 146.05 -63.7a 5.970
Xenon Xe 131.30 -112.0 -108.1 5.367
aSublimation point.
bAt 26 atmospheres. 4He will not freeze below
a pressure of about 20 atmospheres.
General Safety Considerations
The demonstrations in this chapter present special hazards, since most
involve very hot or extremely cold substances, volatile chemicals,
fragile
glassware, high pressure gases or evacuated containers. Procedures
should
be developed to ensure that the demonstration can be done without
danger
to the audience or to one's self. Hot and cold substances should be
handled
with tongs or with special, thermally insulated gloves. Eye protection
should be worn not only to avoid personal injury but to set a proper
example
for the audience. A fire extinguisher, first aid kit, safety shower and
telephone should be available nearby.* The person performing
the demonstrations should know where these are located and how to use
them.
An assistant trained in first aid and CPR provides an extra measure of
protection. If there are children in the audience they should be
cautioned
not to attempt certain of the demonstrations at all and not to do
others
unless a parent or another adult is present. The reason for the
precaution
should be explained to them.
*Available from Lab Safety Supply, P. O. Box 1368,
Janesville,
WI 53547-1368 (608) 754-2354
In the event of a burn, the burned skin should be put under cold
water
until the pain goes away. If blisters are present, they should be
covered
with a sterile gauze. If the burn is severe or if a large area is
involved,
one should see a doctor. With frostbite, the area should be warmed
slowly
with lukewarm (about 106°F) water or with another part of the body,
such as putting a finger under an armpit. Cuts and abrasions should be
cleaned with soapy water, dried and covered with sterile gauze. If
bleeding
is severe, apply pressure to the cut and/or the pulse point above the
cut.
Elevate the cut above the level of the heart. If bleeding persists or
if
the cut is severe, one should see a doctor within a few hours to have
the
cut stitched.
Safety considerations specific to sound, electricity and lasers will
be covered in subsequent chapters.
2.1
Liquid Nitrogen Cannon
The rapid evaporation of liquid nitrogen inside a steel cylinder exerts
enough pressure to blow a cork stopper off the cylinder.
MATERIALS
-
Dewar of liquid nitrogen
-
steel cylinder with smaller, internal, stainless steel cup
-
cork stopper
-
mallet
PROCEDURE
A spectacular demonstration involving the vaporization of liquid
nitrogen
can be done by lowering a small stainless steel cup (~30 milliliters)
suspended
by a wire and filled with liquid nitrogen into a larger steel cylinder
(40 cm long × 5 cm i.d.) sealed on one end. A cork stopper is
pounded
into the other end with a mallet. Then the whole device is picked up
and
shaken in order to spill the nitrogen from the cup. When the liquid
nitrogen
strikes the wall of the cylinder it rapidly evaporates, causing a large
and sudden pressure increase which blows the cork off with great force
and noise. A heavy base on the cylinder helps to absorb the shock of
the
recoil. Liquid nitrogen can be procured by hospital supply sources. If
a Dewar flask is not available, an ordinary thermos bottle can be used
to store the nitrogen if it is made of metal or Pyrex glass. Ordinary
glass can shatter at the low temperature of liquid nitrogen, and the
container should not be sealed tightly lest the thermos
bottle explode as the nitrogen evaporates. If liquid nitrogen is not
available,
a piece of dry ice can be used instead[1].
DISCUSSION
When a liquid evaporates, the resulting gas occupies a much larger
volume
than the liquid from which it came if the pressure is the same in both
cases. The expansion typically amounts to about a factor of 1000 for
most
liquids (compare the density of the gas with the density of the liquid
from which it came). If the gas is not allowed to expand, the pressure
will increase by the same factor. The increased pressure raises the
boiling
point and inhibits the boiling, as in a pressure cooker, but not enough
to prevent a large pressure buildup, as demonstrated here. In the
liquid
nitrogen cannon, only a small fraction of the liquid nitrogen needs to
boil in order to blow the cork off with considerable force.
HAZARDS
Liquid nitrogen boils at a temperature of -196°C (-321°F, 77K)
and can cause severe frostbite. Care should be taken to avoid contact
with
the liquid and with anything that has been cooled by it. Aside from
making
sure the cylinder is quite strong, the only other precaution is to aim
the cannon above the heads of the audience. The cork can travel several
hundred feet, but is relatively harmless after it bounces off a wall or
the ceiling.
REFERENCE
1. J. S. Miller, Physics Fun and Demonstrations, Central
Scientific
Company: Chicago (1974).
2.2
Collapsing Can
A small amount of water is placed in an aluminum, soft-drink can and
brought
to a boil over a Bunsen burner and then inverted in a bath of cold
water,
causing the can to instantly collapse as a result of the rapid
condensation
of the steam.
MATERIALS
-
aluminum, soft drink can
-
15 ml of water
-
Bunsen burner or hot plate
-
large tongs
-
tray of cold water
PROCEDURE
Put about 15 milliliters of water in an empty, 12-ounce, aluminum,
soft-drink
can (one with "Crush" in its name is good) and heat it over a Bunsen
burner
or hot plate until a cloud of condensed water vapor escapes from the
mouth
of the can for about 20 seconds. One should point out that the cloud is
not "steam," which is an invisible gas or smoke which is a collection
of
tiny solid particles. Using tongs, quickly lift the can from the burner
and invert it in a tray containing cold water to a depth of a few
centimeters.
The can will collapse instantly[1,2].
DISCUSSION
When the can cools, the water vapor condenses, reducing the pressure
inside
the can from which most of the air has been expelled. The surface area
of the can is about 0.031 m2 (or 48 in2). In the
most extreme case, a pressure difference of 1 atmosphere (14.7 lb/in2)
would apply a total force of about 700 pounds which is far in excess of
what the can is able to sustain. One might suspect that the water would
be drawn up into the can. However, since the hole in the can is small
and
the condensation occurs quickly, the viscosity of the water inhibits it
from entering the can. Some water does spray up into the can, and it is
this water that causes the steam to condense so rapidly.
HAZARDS
The can contains boiling water at 100°C and can cause burns to the
skin if it is touched with unprotected hands. Be sure the outside of
the
can is clean to prevent it from catching fire.
REFERENCES
1. G. Kauffman, Journ. College Sci. Teach. 14, 364 (1985).
2. B. Z. Shakhashiri, Chemical Demonstrations, The
University
of Wisconsin Press: Madison, Wisconsin, Vol 2 (1985).
2.3
Carbon Dioxide Trough
Carbon dioxide from a glass beaker is poured down a trough containing a
number of candles which are successively extinguished by the invisible
gas.
MATERIALS
-
V-shaped trough with a number of candles mounted inside*
-
2-liter glass beaker
-
cardboard cover for beaker
-
small block of dry ice
-
tongs
-
matches
-
hotplate (optional)
*Available from Frey Scientific Company
PROCEDURE
A V-shaped metal or plexiglas trough inclined at an angle of about
30°
contains about five candles mounted vertically within the trough with
their
wicks below the top of the trough. The candles are lit in honor of
someone's
birthday. There is a better than even chance that someone in the
audience
will have a birthday on any given day if there are at least 250 people
in the audience (see Table 2.2). A 2-liter glass beaker containing
carbon
dioxide gas is removed from behind the lecture table and poured down
the
trough. The candles go out one by one as if water were pouring down the
trough, but the beaker is apparently empty since carbon dioxide is a
colorless,
odorless gas.
The carbon dioxide was produced by placing a piece of dry ice in the
beaker before the lecture with a piece of cardboard on the top to cover
the beaker. The cardboard is removed, and the dry ice is lifted out
with
tongs just before the beaker is shown to the audience. The beaker can
be
stored on a slightly warm, electric hotplate to prevent the formation
of
water ice on its exterior and to ensure the rapid evaporation of the
dry
ice. Dry ice can often be procured from ice cream vendors.
DISCUSSION
This demonstration illustrates the necessity of oxygen for sustaining
combustion.
It also demonstrates sublimation, the process in which a liquid changes
directly from a solid to a gas without going through the liquid phase.
Dry ice is called "dry" because, unlike ordinary water ice, it
sublimates
at atmospheric pressure. Ordinary ice sublimates (and hence is also
dry)
when the vapor pressure of the water above the liquid is below 4.58
torr
(or 0.006 atmospheres). This condition can exist on a dry day and is
the
reason why snow and ice eventually disappear even when the temperature
is below freezing (0°C). Liquid carbon dioxide cannot exist below
the
triple point pressure of 5.1 atmospheres and temperature of
-56.2°C.
The carbon dioxide remains in the beaker because it is about 50% more
dense
than air. Some types of fire extinguishers use carbon dioxide.
HAZARDS
Dry ice sublimates at a temperature of -78.5°C and can cause
frostbite.
Thus it should not be allowed to touch the bare skin.
Table 2.2
Probability that Someone in an Audience of a Given Size Will Have a
Birthday
on a Given Day
Audience Size Probability
10 3%
20 5%
30 8%
70 13%
100 24%
150 34%
200 42%
250 50%
300 56%
400 67%
500 75%
2.4
Exploding Soap Bubbles
Soap bubbles blown with natural gas or hydrogen are ignited with a
candle
as they rise toward the ceiling.
MATERIALS
-
soap bubble solution
-
glass pipe
-
natural gas (methane) or hydrogen
-
candle
-
matches
PROCEDURE
Soap bubbles can be made by filling a glass pipe with a small amount of
soap solution (sold in toy stores). Suitable solutions can also be made
by adding a few drops of sodium or potassium oleate or liquid dish
detergent
to a beaker of warm, distilled or very soft, pure water. A small amount
of glycerine and a few drops of ammonia water greatly improves the
lasting
quality of the bubbles[1].
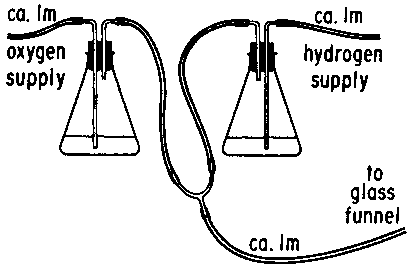 The
pipe is
supported by a ring stand and connected to a source of compressed gas.
A gas lighter than air, such as helium,* allows the bubbles
to rise to the ceiling as they are released from the pipe. The gas
should
be allowed to flow through the pipe briefly before filling it with soap
solution in order to expel the air. If the bubbles are blown with
hydrogen,
they can be ignited with a candle held in the hand as they rise[2,3].
Some
oxygen can be mixed with the hydrogen, using the arrangement shown in
the
figure, to make a much louder explosion. The loudest explosion will
occur
when the hydrogen-to-oxygen ratio has a stoichiometric value of 2:1 so
as to produce pure H2O. Natural gas, often conveniently
available
on the lecture bench, makes a very beautiful and quiet flame. Natural
gas
consists primarily of methane (CH4) which is slightly less
dense
than air. Mixtures of methane and oxygen will rise only if the ratio of
CH4 to O2 is large. The effect is best if viewed
against a dark background in subdued light.
The
pipe is
supported by a ring stand and connected to a source of compressed gas.
A gas lighter than air, such as helium,* allows the bubbles
to rise to the ceiling as they are released from the pipe. The gas
should
be allowed to flow through the pipe briefly before filling it with soap
solution in order to expel the air. If the bubbles are blown with
hydrogen,
they can be ignited with a candle held in the hand as they rise[2,3].
Some
oxygen can be mixed with the hydrogen, using the arrangement shown in
the
figure, to make a much louder explosion. The loudest explosion will
occur
when the hydrogen-to-oxygen ratio has a stoichiometric value of 2:1 so
as to produce pure H2O. Natural gas, often conveniently
available
on the lecture bench, makes a very beautiful and quiet flame. Natural
gas
consists primarily of methane (CH4) which is slightly less
dense
than air. Mixtures of methane and oxygen will rise only if the ratio of
CH4 to O2 is large. The effect is best if viewed
against a dark background in subdued light.
*Specialty gases and equipment can be procured from
Matheson
Gas Products, P. O. Box 1587, Secaucus, NJ 07094.
DISCUSSION
This demonstration illustrates a number of physical concepts. The very
existence of bubbles is a dramatic demonstration of surface tension. A
bubble consists of a thin film of liquid, held together by surface
tension.
It has elastic properties similar to those of a balloon. The size of
the
bubble is determined by the pressure of the gas inside it and the
radius
and thickness of the water film. That the gas inside a bubble is under
pressure can be illustrated by blowing a bubble with a pipe, but before
the bubble is released, allowing it to deflate and blow out a candle
near
the stem of the pipe. A smaller bubble has a higher pressure than a
larger
bubble for the same reason that a balloon is hard to blow up at first
but
becomes easier as it gets larger. This can be illustrated with a
T-shaped
pipe in which two bubbles of different size are blown and then
connected
together by way of the pipe. The larger bubble will get larger, and the
smaller bubble will get smaller.
The tendency of a bubble to rise when filled with a gas lighter than
air illustrates Archimedes' principle. The bubble will rise if its
weight
plus the weight of the gas inside is less than the weight of the air
displaced.
At room temperature and atmospheric pressure, a cubic meter of air
weighs
almost three pounds. A bubble filled with air will slowly drop to the
floor
because of the weight of the film of water. The weight of the air
inside
approximately cancels the weight of the air displaced, neglecting the
small
difference in pressure. From the radius of the bubble and its terminal
velocity as it falls (see the discussion in section 1.4), one can
estimate
the weight of the water and hence the thickness of the water film (the
density of water is 1000 kg/m3). An air-filled bubble can be
made to float in a bath of carbon dioxide produced by dry ice in the
bottom
of a transparent container such as a fish tank.
Bubbles are very fascinating and instructive. Entire lectures can be
given on the properties and behavior of bubbles, and such
demonstrations[4-7]
inevitably enthrall the audience.
HAZARDS
Aside from the normal precautions involved with flames, the oxygen
mixtures
can make quite a loud explosion. The demonstrator should use ear plugs
and caution the members of the audience to hold their hands over their
ears.
REFERENCES
1. C. L. Strong, Scientific American 220, 128 (May 1969).
2. B. Z. Shakhashiri, Chemical Demonstrations, The
University
of Wisconsin Press: Madison, Wisconsin, Vol 1 (1983).
3. H. A. Robinson, ed., Lecture Demonstrations in Physics,
American
Institute of Physics: New York (1963).
4. C. V. Boys, Soap Bubbles and the Forces Which Mould Them,
Educational Services Incorporated, Doubleday Anchor Books: Garden City,
New York (1959).
5. C. Isenberg, The Science of Soap Films and Soap Bubbles,
Tiesto
Ltd.: Clevedon (1978).
6. S. Simon, Soap Bubble Magic, Lathrop, Lee and Shepard:
New
York (1985).
7. A. Ward, Experimenting with Surface Tension and Bubbles,
Dryad
Press: London (1985).
2.5
Hero's Engine
A specially constructed, glass flask containing water and suspended
from
above by a chain spins rapidly when heated from below.
MATERIALS
-
flask with steam tubes (Hero's engine)*
-
Bunsen burner
-
matches
-
water
*Available from Carolina Biological Supply Company, Central
Scientific Company, Edmund Scientific, Fisher Scientific, Frey
Scientific
Company and Sargent-Welch Scientific Company
PROCEDURE
A glass flask with glass tubes connected to the neck and bent to one
side,
suspended by a bead chain, containing a small amount of water and
heated
from below by a Bunsen burner, spins rapidly as the water is brought to
a boil. The glass tubes act as jets releasing the steam in one
direction.
A bead chain provides freedom to rotate since it will not wind up. The
amount of water used is not critical. Enough should be used so that it
doesn't all boil away, but if too much is used, it will take a long
time
to begin to boil. The speed of rotation can be adjusted by varying the
amount of heat provided from below. A more rugged version can be
constructed
using an aluminum cylinder[1]. The device is called "Hero's engine"
after
the Greek philosopher Hero of Alexandria who in the first century B.C.
wrote about it but apparently never actually made one. An analogy could
be made to a rotating lawn sprinkler which can also be demonstrated in
a lecture room using compressed air in place of water.
DISCUSSION
This demonstration illustrates the conservation of angular momentum,
Newton's
third law (action and reaction) and the principle of a rocket engine.
As
the water vapor is expelled, it imparts an equal and opposite momentum
to the flask causing it to rotate in the opposite direction. It
illustrates
the transformation of heat energy into mechanical energy and shows the
large amount of steam that can be produced from a small amount of
water.
Note the great simplicity of this engine as compared to other forms of
steam engines which have many complicated moving parts. Perhaps it
would
be more widely used were it not for the fact that it is enormously
inefficient
at converting heat into motion because the exhaust velocity its
relatively
low and much heat is wasted (exhausted to the air).
HAZARDS
The flask should be of the type that is designed to be heated with a
burner.
Care should be taken not to boil the water too rapidly lest the flask
begin
to spin uncontrollably. The flame should be extinguished before the
water
has completely boiled. The flask should be allowed to cool before it is
touched with unprotected hands.
REFERENCE
1. L. Hirsch, Am. Journ. Phys. 46, 773 (1978).
2.6
Model Geyser
A model geyser consisting of a column of water heated from below with a
Bunsen burner erupts periodically and shoots water up to the ceiling.
MATERIALS
-
model geyser
-
Bunsen burner
PROCEDURE
A working model of a geyser can be rather easily constructed[1]. It
requires
only a long, vertical, water-filled tube with a constriction at the
top,
a supporting structure, a Bunsen burner to heat the tube from below and
a catch basin to collect the water and return it to the tube after an
eruption.
Depending on the length and volume of the tube and the amount of heat
used,
it can be made to erupt every few minutes. A water column of about 2
meters
height works well since it makes the boiling point about 5°C higher
at the bottom than at the top. If the tube is made of pyrex glass, the
activity in the whole geyser tube can be observed. One can contrast the
turbulent nature of the boiling to the regular periodic nature of the
eruptions.
DISCUSSION
The geyser operates by heating the water at the bottom of the tube to a
superheated state (greater than the boiling point of water at
atmospheric
pressure). The water does not immediately boil, however, since the
boiling
point is elevated by the pressure of the water column above. When it
finally
does begin to boil, the pressure of the water column is relieved, and
the
boiling vigorously ensues, causing the eruption. The ejected water
cools
upon contact with the air and the catch basin and runs back down the
tube,
and the heating process begins again.
In addition to the eruption, it should be possible to observe
tremors
of various types as well as a water-hammer effect that occurs just
after
an eruption when the water is momentarily prohibited from running down
the tube by the pressure of the steam remaining in the tube. As the
steam
condenses, a partial vacuum forms in the geyser tube and audibly
"sucks"
the pool of water from the catch basin into the tube with great force.
The resulting noise sounds like a belch and invariably elicits a
reaction
from the audience. A similar effect can be produced by pouring water
into
a funnel inserted through a rubber stopper into an empty flask.
HAZARDS
The geyser is relatively safe if one exercises normal caution in not
touching
the Bunsen burner or the tube containing the hot water. The water can
cause
burns if one gets too close when it erupts, but by the time the water
has
sprayed into the air and fallen back down, it is no more dangerous than
a hot shower. Some water may miss the catch basin and damage other
nearby
equipment.
REFERENCE
1. L. W. Anderson, J. W. Anderegg, and J. E. Lawler, American Journal
of
Science 278, 725 (1978).
2.7
Magdeburg Hemispheres
Two hemispheres when placed together and evacuated cannot be pulled
apart
because of the atmospheric pressure.
MATERIALS
-
Magdeburg hemispheres*
-
vacuum pump
-
pressure gauge (optional)
*Available from Central Scientific Company, Fisher
Scientific
and Frey Scientific Company
PROCEDURE
The large force that the atmosphere can exert is vividly demonstrated
with
a pair of Magdeburg hemispheres, named after the city of Magdeburg in
Germany
where Otto von Guericke (1602-1686), a physicist and politician, while
mayor of the city in 1657, entertained the local people by having two
teams
of eight horses each try to pull apart two, half-meter-diameter, copper
hemispheres held together only by the pressure of the atmosphere[1].
Otto
von Guericke devised one of the first vacuum pumps in about 1641 and
was
largely responsible for dispelling the medieval view that "nature
abhors
a vacuum."
For a more modest demonstration, one can use steel hemispheres with
a diameter of about 12 centimeters and a gasket of some sort to prevent
leakage of air around the joint. If a good vacuum pump is not
available,
one can use larger hemispheres and a water aspirator or hand pump to
produce
a partial vacuum. One of the hemispheres should have a lip around the
edge
to prevent the hemispheres from being slid across the gasket. The
hemispheres
are placed together and evacuated with a vacuum pump through a hose
connected
to a valve on the side of one of the hemispheres. The valve is then
shut
and the hose removed. The hemispheres are usually equipped with handles
so that two volunteers from the audience can try to pull them apart. It
is safe to offer them a sum of money if they succeed (without opening
the
valve!).
In a variation of the demonstration, one can use two disks with the
same projected area as the hemispheres to show that the volume of the
evacuated
space is not the relevant factor. Rubber suction cups of various types
can be shown. A bathroom plunger is a familiar example. A pair of such
plungers can be used as an inexpensive version of the Magdeburg
hemisphere
demonstration.
DISCUSSION
The atmosphere exerts a force of (pi)r2p or 6.25(pi) sq in
×
14.7 pounds/sq in = 290 pounds for a 5-inch diameter sphere completely
evacuated. Even with only a partial vacuum, it is nearly impossible for
two people of normal strength to separate them. One can hang weights
from
one of the hemispheres with the other attached to a secure hook in the
ceiling to get a quantitative measure of the atmospheric pressure. With
Otto Von Guericke's half-meter hemispheres, the force is over two tons!
HAZARDS
Assuming the spheres are made of a material sufficiently strong not to
be crushed by the atmosphere, the only danger is from one of the
volunteers
falling if the other pulls too hard or lets go. A suitable conclusion
to
the demonstration is to open the valve and let air in while the
volunteers
are still pulling, in which case they should be instructed not to pull
too hard.
REFERENCE
1. J. Higbie, Am. Journ. Phys. 48, 987 (1980).
2.8
The Impossible Balloon
A specially constructed balloon appears to have a lifting power far
beyond
what is permitted by Archimedes' principle.
MATERIALS
-
specially constructed balloon
-
weights
PROCEDURE
Professor Ed Miller of the University of Wisconsin likes to perform a
demonstration
involving a buoyancy scam in which a half-meter-diameter balloon is
against
the ceiling with a weight hanging below it. After explaining all about
buoyancy, Professor Miller goes into an enthusiastic spiel about the
exciting
development by some of his colleagues of a new gas with 47 times the
lifting
power of hydrogen and how we are very fortunate to get an advance
demonstration.
Then he hangs several more, obviously heavy weights to the string from
the bottom of the balloon. Eventually someone wakes up and tries to
argue
that this is inconsistent with all he has been saying. He doesn't say a
word, just turns around, reaches up and pulls out the cork to release
the
gas from the balloon. The balloon "flooffs" out its gas and droops down
to nothing, but the weights stay hanging there. Obviously there is a
string
through the balloon to a small hook in the ceiling that holds the
weights
up. Finally a ring on top of the balloon, that kept it steady, falls
down
around the collapsed balloon onto the weights with a clatter as a
humorous
climax to the scam.
DISCUSSION
The amount of weight that a balloon can lift is equal to the weight of
the air displaced by the balloon minus the weight of the balloon and
the
gas that it contains (Archimedes' principle). Thus hydrogen (H2)
with a molecular weight of 2 grams per mole compared to air with an
average
molecular weight of 29 grams per mole is close to the best that one can
do. Even a gas with zero molecular weight (a vacuum) could provide a
lift
only 7% greater than hydrogen. The use of an evacuated balloon is, of
course,
impractical since the balloon would be much too heavy if made of a
material
sufficiently strong to support the pressure of the atmosphere. The
pressure
in a hydrogen-filled balloon is actually slightly greater than the
pressure
of the surrounding atmosphere (typically 5-10%), and so such a balloon
would contain more moles of hydrogen than the air that is displaced,
which
reduces its buoyancy slightly.
HAZARDS
The only hazard in this demonstration is the string breaking and
dropping
the weights on one's foot.
2.9
Boiling with Ice
Water in a sealed flask is made to boil by inverting the flask and
holding
an ice cube against its bottom.
MATERIALS
-
1-liter, thick-walled, round-bottomed boiling flask
-
two-hole rubber stopper
-
thermometer
-
glass tube
-
rubber hose with pinch clamp
-
Bunsen burner
-
ice cube
PROCEDURE
Water can be made to boil at a temperature well below 100°C by
reducing
the pressure of the atmosphere above the water[1-5]. A 1-liter,
thick-walled,
round-bottomed boiling flask is filled about half way with water. A
two-hole
rubber stopper is used to seal the flask. A thermometer is placed in
one
hole of the stopper. In the other hole is a glass tube connected to a
rubber
hose which can be sealed with a pinch clamp. The water is brought to a
boil with a Bunsen burner. The water is then allowed to cool for one
minute.
The pinch clamp is then used to seal the flask, the flask is inverted,
and an ice cube is held against its bottom. The water in the flask will
boil for several seconds.
DISCUSSION
The temperature at which a liquid boils depends upon the pressure of
the
atmosphere above it. The water will boil when its vapor pressure (see
Table
2.3) exceeds the pressure of the surrounding gas. In this
demonstration,
the pressure is lowered from normal atmospheric pressure (760 torr) by
cooling the air in the flask and by condensing some of the water vapor
in the flask. As the water boils, the pressure in the flask increases
to
the point where it is greater than the vapor pressure of the liquid,
and
the water stops boiling. The flask should be no more than half full of
water to provide space for the vapor produced by boiling. If too little
space is provided, the vapor pressure of the liquid water may be
reached
before boiling is apparent.
HAZARDS
In addition to the hazard of burns from the Bunsen burner and the hot
flask,
the flask may implode if the pressure is lowered sufficiently. This
risk
is minimized by using a thick-walled boiling flask rather than a
standard
round-bottomed one.
REFERENCES
1. D. Baisley, The Science Teacher 47, 45 (May 1980).
2. A. Joseph, P. F. Brandwein, E. Morholt, H. Pollack and J. F.
Castka,
A Sourcebook for the Physical Sciences, Harcourt, Brace and
World:
New York (1961).
3. H. A. Robinson, ed., Lecture Demonstrations in Physics,
American
Institute of Physics: New York (1963).
4. B. Z. Shakhashiri, Chemical Demonstrations, The
University
of Wisconsin Press: Madison, Wisconsin, Vol 2 (1985).
5. J. P. VanCleave, Teaching the Fun of Physics, Prentice
Hall
Press: New York (1985).
Table 2.3
Temperature Dependence of the Vapor Pressure of Water
Temperature (°C) Pressure (torr)
0 4.579
5 6.543
10 9.209
15 12.788
20 17.535
25 23.756
30 31.824
35 42.175
40 55.324
45 71.88
50 92.51
55 118.04
60 149.38
65 187.54
70 233.7
75 289.1
80 355.1
85 433.6
90 525.76
95 633.90
100 760.00
2.10
Freezing by Evaporation
Water at room temperature in a flask boils vigorously and then turns
into
ice when the pressure in the flask is reduced.
MATERIALS
-
thick-walled, round-bottom boiling flask
-
rubber vacuum hose with valve
-
sulfuric acid trap
-
vacuum pump
-
distilled water
-
pressure gauge (optional)
PROCEDURE
With the use of a mechanical vacuum pump, water in a flask can be made
to boil so vigorously at room temperature that the loss of heat due to
evaporation causes the water to freeze[1]. Apparatus for such a
cryophorus
demonstration can be purchased or constructed.If constructed, one
should
use a thick, round-bottom boiling flask and a trap which can be filled
with strong sulfuric acid to isolate the flask from the vacuum pump.
The
acid absorbs the water vapor thereby increasing the rate of boiling as
well as preventing the pump oil from being contaminated with water. The
use of distilled water is recommended. The water can be brought to a
boil
more quickly if it is preheated. A large pressure gauge visible to the
audience is a useful addition.
This demonstration is best introduced by asking the audience at what
temperature water will boil. Most people will answer with 212°F or
100°C. One should point out that this answer is correct only at
atmospheric
pressure (760 torr), and that it is well-known by the residents of
high-altitude
cities that it takes longer to cook a boiled egg there because water
boils
at a lower temperature at higher altitude. It's not accurate to say
that
it takes longer to boil an egg there because the water is actually
brought
to a boil more quickly. The barometric pressure drops by about 3% for
each
1000 feet above sea level. A city such as Denver, Colorado is at an
altitude
of about 5000 feet, and thus the average barometric pressure is around
660 torr, and the boiling point of water is about 96°C. On top of
Mt.
Everest (29,028 feet) it's a chore to make a pot of tea. One can point
out that a boiling liquid tends to maintain a constant temperature, and
this is why many foods are cooked in boiling water. In a pressure
cooker,
the boiling point is raised by the increased pressure.
One then turns on the vacuum pump and lets the audience watch while
the water boils furiously. One can touch the flask and point out that
it
isn't hot at all, and, in fact, if anything, it feels a bit cool. While
holding a hand on the flask, ask the audience at what temperature water
will freeze. If this is timed properly, the water will freeze on cue
into
a slushy form of ice. In a large hall, the ice can best be seen in
silhouette
on a screen illuminated by an arc lamp or by a slide projector.
DISCUSSION
The heat of vaporization of water is 540 calories per gram, and the
heat
of fusion of water is 80 calories per gram. The density of water is 1
gram
per milliliter. When the water evaporates, a large amount of heat is
thus
extracted from the water, causing the cooling. The evaporation of each
milliliter of water is capable of freezing almost seven milliliters
once
the temperature of the water has decreased to 0°C. As the
temperature
drops, the vapor pressure of the water drops, and the boiling is
greatly
reduced. This heat loss is why we feel cool when we get out of the bath
or swimming pool. Animals perspire in order to keep cool by the same
process.
If our perspiration evaporated too rapidly, we would freeze to death!
HAZARDS
The major hazard is implosion of the flask if it is not designed to
withstand
atmospheric pressure or if it is dropped or struck with a heavy object.
Sulfuric acid can also cause burns and should be handled with great
caution.
REFERENCE
1. H. A. Robinson, ed., Lecture Demonstrations in Physics,
American
Institute of Physics: New York (1963).
2.11
Nonburning Handkerchief
A cotton handkerchief is doused in a liquid and set on fire, but the
handkerchief
doesn't burn.
MATERIALS
-
cotton handkerchief or dollar bill
-
50% solution of isopropyl alcohol and water
-
small amount of salt (sodium chloride)
-
matches or cigarette lighter
-
tongs, at least 30 cm long
-
bucket of water (recommended)
-
coin and cigarette (optional)
PROCEDURE
A brightly colored, cotton handkerchief is taken from the pocket, or
better
yet, from a volunteer planted in the audience and is soaked in a liquid
and set on fire[1]. While holding the handkerchief with tongs and
watching
the blue flame, one points out that the handkerchief is not burning.
Finally
the handkerchief is snuffed out with a quick jerk or doused in a bucket
of water and passed around for inspection or returned to the volunteer.
For more drama, an assistant can put out the flame with a fire
extinguisher.
The volunteer can be asked to hold up the handkerchief for everyone to
see.
In this demonstration, the liquid used is a 50/50 mixture of
isopropyl
alcohol and water. Methanol or ethanol can be substituted for the
isopropyl
alcohol. Some salt in the solution will help to make the flame more
visible.
Other combustible materials, such as paper, can also be used in place
of
the handkerchief[2]. A dollar bill provided by someone in the audience
is especially effective. Note carefully the purity of the alcohol
before
mixing. Rubbing alcohol as sold in drug stores is often 30% or more
water.
One could repeat the demonstration with varying mixtures of alcohol and
water. Too much water will prevent the alcohol from burning, and too
little
water will allow the cloth or paper to char.
In a variation of the demonstration, a dry cotton cloth is wrapped
tightly
around a coin, and a lighted cigarette is touched to the cloth. The
coin
absorbs the heat and keeps the temperature below the point where the
cloth
burns.
DISCUSSION
This demonstration illustrates the variation in the temperature
required
to support combustion in different substances. The alcohol burns at a
temperature
below the kindling temperature of the cotton. In addition, the heating
and vaporization of the water removes heat and prevents the cloth from
burning.
HAZARDS
Although the flame is relatively cool, it is capable of producing
severe
burns. Hold the handkerchief with a pair of very long tongs (30 cm or
more)
while it is ignited. Plan ahead to have a way to extinguish it before
the
water is completely vaporized or if it inadvertently drops to the floor
while burning. Isopropyl alcohol can damage the eyes severely.
REFERENCES
1. B. Z. Shakhashiri, Chemical Demonstrations, The University
of
Wisconsin Press: Madison, Wisconsin, Vol 1 (1983).
2. J. Jardin, P. Murray, J. Tyszka, and J. Czarnecki, Journ. of
Chemical
Education 55, 655 (1978).
2.12
Liquid Nitrogen Cloud
Liquid nitrogen induced to vaporize rapidly by expelling it from a
large
Dewar under pressure cools the air and causes the formation of a large,
dense cloud.
MATERIALS
-
25-liter Dewar filled with liquid nitrogen
-
2-hole rubber stopper to fit Dewar
-
specially constructed copper pipe with exhaust holes
-
compressed air or nitrogen gas
-
electrical heating tapes (optional)
PROCEDURE
An impressive cloud can be produced by the rapid boiling of liquid
nitrogen.
Suitable apparatus consists of a large (25-liter) Dewar filled with
liquid
nitrogen. A two-hole rubber stopper plugs the mouth of the Dewar. In
one
hole of the stopper is placed a tube connected to high-pressure air or
through a pressure regulator to a cylinder of compressed nitrogen. In
the
other hole is placed a tube that leads to the center of a meter-long,
thick-walled,
horizontal, copper pipe, sealed at the ends, with a dozen or more small
(1/8" diameter) holes in the top through which the nitrogen can escape
into the room. Electrical heating tapes surrounding the pipe can be
used
to warm the pipe prior to use. If a pipe with sufficiently thick walls
is unavailable, a solid cylinder of copper can be fit loosely inside
the
pipe. The aim is to provide a warm orifice of high heat capacity to
cause
rapid boiling of the nitrogen for as long as possible. Depending upon
the
heat capacity of the pipe and the flow rate of gas into the Dewar, a
cloud
reaching from the floor up several meters into the air can be sustained
for a good fraction of a minute until the pipe cools to too low a
temperature.
The demonstration cannot be repeated for about an hour or so because
the
pipe has to warm back up to room temperature.
The operation of the device involves forcing the liquid nitrogen
from
the Dewar up into the warm pipe where it rapidly boils and exits from
the
holes in the pipe as cold nitrogen gas. The cold gas then condenses
moisture
from the air in the room above the apparatus and forms the cloud. Thus
the demonstration works best when the humidity is high. If there is a
ventilation
system in the room, it can be turned off a few minutes before the
demonstration
to allow the humidity to rise. After 30 seconds or so the pipe will
cool
to the point where fountains of liquid are emitted from the holes. The
liquid is not nearly so effective as the cold gas in cooling the air,
and
the cloud slowly subsides. Colored lights illuminating the cloud
provides
extra visual appeal. The demonstration is an effective conclusion to a
presentation, and the demonstrator can disappear in the cloud and
perhaps
exit the room through a nearby door unseen by the audience.
DISCUSSION
Although this demonstration is done mostly for the drama, a number of
physical
principles are illustrated including Pascal's law, heat of
vaporization,
heat capacity, heat transport, and the condensation of water vapor in
air
as the temperature is lowered. The mechanism is similar to the way
clouds
in the sky are formed by cooling of the air to a temperature below
which
the air becomes saturated with moisture (100% relative humidity). The
cloud
consists of extremely small droplets of liquid water and is not steam
or
smoke as many people will respond if asked.
HAZARDS
A possible hazard is explosion of the Dewar due to over-pressurization.
The exit holes should not be obstructed, and pressures higher than
about
30 psi should not be used unless the Dewar is known to be of adequate
strength.
It is wise to test the Dewar behind a suitable barricade at a pressure
about 50% greater than what one intends to use. Some liquid nitrogen
will
exit from the holes especially after the device has run for a while,
and
thus to avoid frostbite, the face and other parts of the body should
not
be directly above the holes in the pipe when it is turned on.
2.13
Heat Transmitter
A match at the focal point of a parabolic reflector is ignited by the
radiation
from an electrical heating element placed at the focal point of a
second
parabolic reflector across the room and aimed at the first.
MATERIALS
-
2 parabolic reflectors*
-
electrical heating element
-
wooden match or gunpowder
-
piece of paper (for alignment)
*Available from Central Scientific Company, Edmund
Scientific,
Fisher Scientific, Frey Scientific Company and Sargent-Welch Scientific
Company
PROCEDURE
The transfer of heat by radiation can be effectively demonstrated with
two parabolic reflectors preferably of 20 centimeters or more diameter.
Near the focus of one reflector is placed an electrical resistive
heating
element. At the focus of the other reflector is placed a wooden match.
The reflectors are then placed several meters apart and aimed at one
another.
When the heating element is energized, the match should burst into
flame
in a few seconds. One can show that it is safe to stand in the beam,
since
the power flux (watts per square meter) is quite low. The reflectors
need
not be of optical quality since the heat source is quite spread out,
but
the surfaces should reflect infrared radiation with high efficiency.
Chrome
plating of the reflectors is helpful. A less effective variation uses a
single reflector the focus an image of the lamp on the match[1]. A
piece
of paper can be used in a dimly lit room to view the red spot on which
the heat is focused to place the match in the optimal location. Avoid
bumping
either of the reflectors after they have been aligned. For a bit more
drama
the match can be replaced with a small amount of gunpowder as Sir
Humphry
Davy (1778-1829) was fond of doing in his public lectures at the Royal
Institution in London[2].
DISCUSSION
Radiation is one of the three ways in which heat can be transferred.
The
others are conduction and convection. Only radiation can take place in
a vacuum. The others require a material medium. This is the means by
which
the sun heats the earth. Heat radiation is a form of electromagnetic
wave
in the infrared portion of the spectrum. It is of the same form as
light,
microwaves, radio waves, X-rays, and so forth, except of a different
wavelength.
When the temperature of a body increases, it emits successively shorter
and shorter wavelengths, first giving off heat and then light. Most
materials
melt before they can give off significant amounts of ultraviolet. The
amount
of energy radiated by a body is proportional to the fourth power of its
absolute temperature (Stefan's law). Infrared radiation obeys the same
laws of geometrical optics as does light, and thus it can be focused by
mirrors and lenses as in this demonstration. Radiation can also be
described
in terms of the emission and absorption of individual photons.
HAZARDS
The heating element can cause burns and electrocution if operated
directly
from the power lines. A bad burn can result if any part of the body or
clothing is placed in the focus of the receiving reflector. Don't try
to
align the reflectors by placing the eye at the focus; rather, observe
the
spot on a piece of paper. A means should be provided to safely
extinguish
the match once it is lit and to avoid dropping the match onto something
that might catch fire. Experiment with small amounts of gunpowder to
get
an explosion that is impressive but safe.
REFERENCES
1. R. E. Berg, Phys. Teach 28,56 (1990).
2. J. Tyndall, Heat; a Mode of Motion, third edition,
Longman:
London (1868).
2.14
Ethanol Vapor Explosion
A small amount of ethanol placed in a bottle is made to explode and
blow
a cork a considerable distance by means of an electrical spark.
MATERIALS
-
250-ml polyethylene bottle with two nails through the sides
-
cork (or rubber stopper ) to fit bottle
-
small quantity of >95% ethanol
-
hand-held Tesla coil* or other high voltage source
*Available from Fisher Scientific and Sargent-Welch
Scientific
Company
PROCEDURE
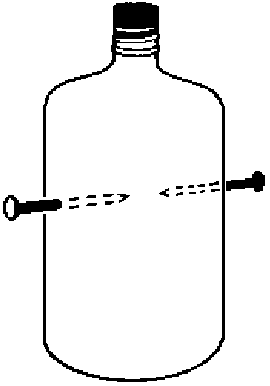 Two to
three
milliliters of >95% ethanol (C2H5OH) is placed
in
a 250-milliliter polyethylene bottle with two nails or screws through
the
sides as shown in the illustration[1]. The tips of the nails or screws
are pointed and come within a few millimeters of touching. The bottle
is
sealed with a cork or rubber stopper. The bottle can be shaken briefly
to ensure that the liquid ethanol has come to equilibrium with its
vapor.
The nails are then connected to a high-voltage (>1000 volts),
low-current
source. The resulting spark ignites the ethanol and blows the stopper
off.
Alternately, a hand-held Tesla coil can be held near one of the nails.
Even with the second nail disconnected, there is enough capacitance
between
the nails to cause a spark to jump across the gap. The capacitance can
be increased, causing a more intense spark, by connecting the second
nail
to ground or to any conductor with a large surface area. A number of
such
bottles can be placed in series electrically, causing all the stoppers
to blow off at the same time. Larger bottles can be used for more
impressive
explosions. One can point out that cars can use ethanol as fuel and
that
the reaction in the cylinders of the engine is the same as the one
illustrated
here.
Two to
three
milliliters of >95% ethanol (C2H5OH) is placed
in
a 250-milliliter polyethylene bottle with two nails or screws through
the
sides as shown in the illustration[1]. The tips of the nails or screws
are pointed and come within a few millimeters of touching. The bottle
is
sealed with a cork or rubber stopper. The bottle can be shaken briefly
to ensure that the liquid ethanol has come to equilibrium with its
vapor.
The nails are then connected to a high-voltage (>1000 volts),
low-current
source. The resulting spark ignites the ethanol and blows the stopper
off.
Alternately, a hand-held Tesla coil can be held near one of the nails.
Even with the second nail disconnected, there is enough capacitance
between
the nails to cause a spark to jump across the gap. The capacitance can
be increased, causing a more intense spark, by connecting the second
nail
to ground or to any conductor with a large surface area. A number of
such
bottles can be placed in series electrically, causing all the stoppers
to blow off at the same time. Larger bottles can be used for more
impressive
explosions. One can point out that cars can use ethanol as fuel and
that
the reaction in the cylinders of the engine is the same as the one
illustrated
here.
DISCUSSION
In this demonstration, some of the liquid ethanol evaporates and mixes
with the air in the bottle. The reaction that takes place is C2H5OH
+ 3O2 --> 2CO2 + 3H2O. Thus there
is an
increase both in the number of moles of gas (4 to 5) and in its
temperature
as a result of the energy released in the exothermic reaction. The
increased
pressure causes the stopper to blow off the bottle. The demonstration
cannot
be repeated without flushing the bottle, because the explosion consumes
all the oxygen in the bottle.
HAZARDS
The stopper is expelled with considerable force, and thus care should
be
taken to avoid damage to light fixtures or other objects. Keep the area
above the bottle clear of obstructions. The voltage used to produce the
spark should be either of a high frequency, as with the Tesla coil, or
a low current to avoid electrocution.
REFERENCE
1. B. Z. Shakhashiri, Chemical Demonstrations, The University
of
Wisconsin Press: Madison, Wisconsin, Vol 2 (1985).
2.15
Heat Convection
A candle is extinguished when a tightly-fitting glass cylinder is
placed
over it unless a T-shaped piece of metal is lowered into the cylinder.
MATERIALS
-
candle
-
glass tube, 30-cm long × 3-cm diameter
-
metal tee
-
matches
-
coil of copper wire (optional)
PROCEDURE
The convection of heated air can be demonstrated quite simply and
effectively
with a candle[1]. The candle is lit to show the audience that it is
quite
ordinary. Then a glass cylinder, about 30 cm long and 3 cm in diameter,
open at both ends, is lowered over the candle, making a tight seal at
the
base so that air cannot be drawn up from below. After a few seconds,
the
candle will go out. Then a thin, tightly-fitting partition made out of
copper or some similar metal is lowered into the cylinder to within a
few
centimeters of the top of the candle. The partition can be held in
place
by a T-shaped extension at its top. The cylinder is lifted, the candle
relit, and the cylinder put back over the candle. The candle continues
to burn with a flickering flame while the cylinder is in place.
Finally,
after awhile, the partition is removed, and the candle goes out. For
each
case, the audience can be asked to predict whether the candle will
continue
to burn. With a bit of practice, one can let the candle apparently go
out
and then revive it by quickly lowering the partition.
As a frill, the candle can also be extinguished by abruptly touching
a coil of copper wire to the flame[2]. The copper conducts the heat
away
and lowers the temperature below the kindling point of the candle wax.
DISCUSSION
In the absence of the partition, the upward rising warm air interferes
with the downward falling cool air near the top of the cylinder, and
the
convection pattern does not reach down to the level of the candle. With
the partition in place, the warm air rises up one side of the partition
and the cool air falls down the other side and replenishes the candle
with
oxygen. Whether the convection goes clockwise or counter-clockwise
around
the partition depends on small asymmetries when the convection starts.
Once the convection starts in one direction, it will tend to continue
in
that direction as long as the candle burns, much as the direction of
the
swirling water leaving the drain in a bathtub depends mainly on the
small
vorticity present in the water when the plug is pulled.*
*The coriolis force due to the rotation of the earth will
determine the direction of the swirl if the initial vorticity is
sufficiently
small. This force is in opposite directions in the northern and
southern
hemisphere.
Along with radiation and conduction, convection is one of the ways
in
which heat is transported from one place to another. Convection can
occur
in gases and liquids. The convection is driven by pressure gradients in
the fluid. With the candle, the pressure gradient is small, and so the
warmer air is less dense than the colder air and thus is not as
strongly
attracted to the earth by gravity. The burning of a candle requires
gravity
as can be demonstrated by observing that a candle in free-fall will
extinguish[3].
HAZARDS
The hazards in this demonstration are rather obvious and include burns
from the candle and cuts from the glass.
REFERENCES
1. J. S. Miller, Physics Fun and Demonstrations, Central
Scientific
Company: Chicago (1974).
2. T. L. Liem, Invitations to Science Inquiry, Ginn Press:
Lexington,
Massachusetts (1981).
3. B. Z. Shakhashiri, Chemical Demonstrations, The
University
of Wisconsin Press: Madison, Wisconsin, Vol 2 (1985).
2.16
Exploding Balloons
Helium and Hydrogen-filled balloons tethered by strings above the
lecture
bench are burst with a match on the end of a stick.
MATERIALS
-
two identical balloons
-
20 feet of thin string
-
helium and hydrogen gas
-
wooden stick about 1 meter long with clip on one end
-
matches
PROCEDURE
Two identical balloons are filled, one with helium, the other with
hydrogen.
The balloons are attached to either end of the lecture bench with thin
strings that allow them to float about ten feet above the floor. The
balloons
should be filled within about an hour of being used since the gas will
gradually diffuse through the balloons, and they will lose their
buoyancy.
The audience is asked to guess what is in the balloons. Most people
will assume they are filled with helium. The audience is then asked to
predict what will happen when a lighted match is touched to one of the
balloons. Most people will say the balloon will burst.
A match is lit and attached with an alligator clip on the end of a
wooden
stick about a meter long. The match is touched to the helium balloon
causing
it to burst. The members of the audience are then asked if they would
like
to see it repeated with the other balloon. One then repeats the
demonstration
with the hydrogen balloon, causing a large explosion and ball of flame.
When the audience regains its composure, one can explain the
importance
of taking nothing for granted in science. Helium is only one example of
a gas that is lighter than air. In fact, hydrogen is lighter yet (about
half as heavy). The upward buoyant force, however, is only slightly
different
since it is given by the difference between the weight of the gas in
the
balloon and the weight of the air displaced by the balloon. On can
proceed
to discuss Archimedes' principle, chemical combustion, or the
scientific
method. Mention of the Hindenburg and the reasons hydrogen is no longer
used in dirigibles is appropriate. The operation of hot-air balloons
can
be discussed.
The demonstration can also be done using other gases such as air and
oxygen if the balloons are suspended in some way such as with a ring
stand.
A stoichiometric mixture (2:1) of hydrogen and oxygen can also be used
if proper precautions are taken to protect one's ears from the loud
explosion
that results[1]. If hydrogen gas is not available, it can be produced
in
a 250 ml pyrex Erlenmeyer flask to which 10 to 15 grams of mossy zinc
and
65 ml of 3M hydrochloric acid has been added[2]. One can increase the
amount
of sound released in the explosion of the hydrogen balloon by putting
some
air in the balloon before it is filled with hydrogen. Experiment to get
an appropriately dramatic but not dangerously loud explosion.
DISCUSSION
The tendency of a balloon to rise when filled with a gas lighter than
air
illustrates Archimedes' principle. The balloon will rise if its weight
plus the weight of the gas inside is less than the weight of the air
displaced.
At room temperature and atmospheric pressure, a cubic meter of air
weighs
almost three pounds. An air-filled balloon or soap bubble can be made
to
float in a bath of carbon dioxide produced by dry ice in the bottom of
a transparent container such as a fish tank.
The combustion of the gas in a balloon is an illustration of a
chemical
reaction, in this case hydrogen in the balloon reacting with oxygen in
the surrounding air to form water with the release of 232 kJ/mole of
water
formed. The reaction ensues much more violently if oxygen is mixed with
the hydrogen than if it has to mix with oxygen from the air. This gives
some indication of the rate with which gases diffuse when the partition
between them (the balloon) is suddenly removed.
HAZARDS
This demonstration looks more dangerous than it is. If the area around
and especially above the balloon is clear of obstructions and if the
balloon
is ignited using a stick at least a meter long at arm's length, it is
relatively
safe. One should practice with small balloons to gain confidence.
Remember
that the volume of hydrogen is proportional to the cube of the diameter
of the balloon, and thus the demonstration quickly scales up to quite
dramatic
proportions. Hydrogen-oxygen mixtures require ear protection for both
the
demonstrator and the audience.
REFERENCES
1. B. Z. Shakhashiri, Chemical Demonstrations, The University
of
Wisconsin Press: Madison, Wisconsin, Vol 1 (1983).
2. S. Isom and C. Lail, The Science Teacher 56, 45 (March
1989).
2.17
Smoke Rings
A cardboard box with a hole in one side is used to produce smoke
vortices.
MATERIALS
-
cardboard box
-
rubber or plastic sheet
-
source of smoke
-
spotlight (optional)
-
candle (optional)
PROCEDURE
One of the six faces of a cardboard box of arbitrary size is removed
and
replaced with a sheet of thin rubber or plastic. A plastic shower
curtain
or even a sheet of cloth will suffice. The sheet is taped to the box to
make as nearly an air-tight fit as possible. A circular hole about 10
cm
in diameter is cut in the cardboard on the side of the box opposite the
sheet. The box is filled with smoke. A good way to do this is to paint
the rim of the hole with an aqueous solution of titanium tetrachloride
(TiCl4) using a cotton swab. Alternately the box can be
filled
with smoke from the combustion of some suitable material or, perhaps
more
safely, with chalk dust or talcum powder. If a spotlight or other
bright
light is available, it should be aimed at the hole in the box from
across
the room.
The box is placed on its side, and the side with the sheet is given
a tap with the hand as one would beat a drum. A smoke ring vortex
should
be emitted from the hole and travel across the room. The process can be
repeated as often as desired so long as some smoke remains. After a
while
the whole room becomes smokey, and visibility is impaired. The first
few
rings are usually the most dramatic. A fast-moving ring can be produced
immediately after a slower one so that it catches up and overtakes the
slower one. Two such vortex generators can be constructed and the rings
projected toward one another to study their interaction. It should be
pointed
out that the vortices are there even in the absence of the smoke, whose
sole purpose is to render them visible. This can be illustrated by
blowing
out a candle from across the room.
The construction of such a vortex generator makes an ideal home
project
for people of all ages. The audience can be encouraged to try making
one
and to investigate the effect of using holes of different sizes and
shapes.
Would a square hole make a square ring? What happens when the ring is
projected
upward?
DISCUSSION
The mechanism whereby the vortex ring is generated is easy to
understand.
When the air exits the hole, it is retarded by friction with the rim of
the hole causing the air in the center to move forward faster than the
air at the edge. If you imagine riding along with the vortex, the air
at
the center is moving forward and the air at the edge is moving
backwards.
This leaves a region of reduced pressure in front of the ring at the
edge
and behind the ring in the center. The extra air in the center front
curves
outward, and the extra air at the edge in back curves inward to
equalize
the pressure thus forming the ring.
Vortex rings are quite a common occurrence in nature. Flowing
liquids
form vortices when they flow too fast down a narrow channel as can be
seen
by watching a fast-flowing river. Vortices off the wing tips of
fast-moving
aircraft are a hazard to other planes that inadvertently fly through
them.
Most people have seen someone who smokes make vortex rings by shaping
the
mouth in the form of a circle and exhaling gently. It was once thought
(incorrectly) that the interaction of atoms and their spectral emission
could be understood in terms of vortex motion inside the atom.
HAZARDS
The hazards are mostly in producing the smoke. One should not place
ignited
materials inside the box. When blowing out a candle from across the
room,
the candle should be kept well away from anything flammable.
2.18
Bell Jar
Object placed in a bell jar connected to a vacuum pump expand when
evacuated
and contract when air is readmitted.
MATERIALS
-
bell jar*
-
vacuum pump
-
balloon
-
carbonated beverage, marshmallows or shaving cream (optional)
*Available from American Scientific Products, Carolina
Biological
Supply Company, Central Scientific Company, Fisher Scientific, Frey
Scientific,
Nasco and Sargent-Welch Scientific
PROCEDURE
A balloon is inflated with air to about half the diameter of the inside
of the bell jar and tied off. It is placed inside the bell jar. The
audience
is asked to predict what will happen when the air is removed from the
bell
jar. A vacuum pump is turned on, evacuating the bell jar. The balloon
expands.
Then the audience is asked to predict what will happen when air is
readmitted
to the bell jar. The pump is turned off, and air is admitted, causing
the
balloon to return to near its original size. The procedure can be
repeated
with other objects such as a carbonated beverage, marshmallows or
shaving
cream[1]. When done with a liquid, a sulfuric acid trap should be used
to prevent the pump oil from becoming contaminated.
DISCUSSION
The size of a balloon is determined by an equilibrium between the
pressure
of the internal gas and the combined effects of the pressure of the
external
gas and the elasticity of the balloon material[2]. The balloon expands
when the external pressure is reduced and contracts when the external
pressure
is increased. A balloon with a diameter of six inches at sea level has
a total external force in excess of three tons! Consequently, even a
partial
vacuum will significantly change its size. The balloon may not return
to
quite its original size because the balloon material may become
permanently
deformed when it expands. It is for this reason that a balloon is
easier
to inflate the second time than the first. A balloon is also easier to
inflate if it is stretched first.
A marshmallow can be considered as a large number of very small
balloons.
Most people know a marshmallow is mostly air. If the marshmallow is
left
in the evacuated bell jar for a few minutes, the air will mostly leak
out
of the trapped pockets, and it will be compressed to about half its
original
size when air is readmitted quickly enough that the pockets don't
refill.
With a carbonated beverage, the carbon dioxide comes out of solution
because
of the reduced pressure of the external gas. The liquid will begin to
boil
if the pump is left on too long.
HAZARDS
The main hazard with this demonstration is implosion of the bell jar
which
has many tons of atmospheric pressure on it while evacuated. Use only
bell
jars designed to be evacuated, and be careful not to drop the bell jar
or strike it with any hard objects.
REFERENCES
1. J. D. Wilson, College Physics, Allyn and Bacon: Needham
Heights,
MA (1989).
2. J. Walker, Scientific American 261, 136 (Dec 1989).
2.19
Liquid Nitrogen
Objects placed in liquid nitrogen change their physical properties
because
of the reduced temperature.
MATERIALS
-
Dewar of liquid nitrogen*
-
plastic hose or rubber ball
-
banana
-
tongs
-
gloves
-
hammer
-
glass dish
-
balloon
*Liquid nitrogen is available from hospital supply houses.
An
ordinary thermos bottle serves as an adequate Dewar if it is made of
metal or Pyrex glass (ordinary glass can shatter).
PROCEDURE
A bit of liquid nitrogen is poured onto the table, and the audience is
asked to note that the table didn't get wet. The substance that looks
like
water is the major constituent (80%) of the air that we breathe, but at
a reduced temperature of -196°C (-321°F, 77K). It is probably
the
coldest substance most people have ever seen. One can explain that it
boils
without evident heating because even ordinary materials like the table
have quite a lot of heat, and not much heat is needed is boil nitrogen.
One explains that most materials change their physical properties
when
they get cold. Most people have noticed how hard the seats of their
cars
are on a cold morning. A plastic hose it dipped into the Dewar of
liquid
nitrogen until the boiling stops and then shattered with a hammer.
While
the nitrogen is boiling, a small cloud appears above the Dewar. The
audience
can be asked what it is. Many will say "steam" or "smoke," but of
course
steam is invisible and smoke is the product of combustion. The cloud
actually
consists of small particles of liquid water condensed from the air
above
the Dewar by the cooling effect of the boiling of the nitrogen.
One can take a rubber ball, bounce it a few time on the floor and
then
drop it into the liquid nitrogen. When the boiling stops, the ball is
removed
with tongs, and then, while wearing thermally insulating gloves, throw
it to the floor causing it to shatter. Alternately, a banana is left in
the liquid nitrogen for a few minutes and then used to drive a large
nail
into a block of styrofoam. The banana has a tendency to shatter and
leaves
quite a mess when it thaws.
A balloon is blown up to about six inches in diameter, tied off and
placed in a glass dish. Liquid nitrogen is poured over the balloon
causing
it to shrivel to negligible size. It is then removed from the dish with
tongs or gloves and held while it slowly reinflates. If one looks
carefully,
it is possible to see some liquid air inside the balloon. The audience
can be asked to explain what is happening.
DISCUSSION
The temperature of liquid nitrogen is suffiently low that most
materials
have significantly different and sometimes surprising properties. Few
elastic
materials retain their elasticity at this temperature. Electrical
resistance
is much lower, and some materials in fact become superconductors and
lose
all electrical resistance.
When the air in a balloon is cooled, its pressure decreases, and the
pressure of the external atmosphere crushes it. Some of the nitrogen
and
most of the oxygen in the balloon may liquefy.
HAZARDS
Liquid nitrogen is potentially very dangerous since it can cause
instant
frostbite. Never allow it to come into contact with bare skin. Objects
should be lowered into the liquid nitrogen and removed with tongs. Be
careful
that it doesn't splash into the eyes. Gloves and protective eyewear are
recommended. When objects are shattered with a hammer or by throwing
them
against the floor or wall, the resulting projectiles can easily injure
someone unless proper precautions are taken.
2.20
Kinetic Theory Simulator
A collection of small ball bearings in an enclosure one side of which
is
connected to a loudspeaker exhibit motion analogous to the molecules in
a gas.
MATERIALS
-
kinetic theory simulator*
*Commercial versions, sometimes called "molecular motion
demonstrators"
are available from Central Scientific Company, Carolina Biological
Supply
Company, Frey Scientific, PASCO Scientific, and Sargent-Welch
Scientific.
PROCEDURE
A dozen or more small plastic or steel balls are placed in a
transparent
container, one boundary of which is made to oscillate rapidly back and
forth by a vibrator or loudspeaker connected to a high power audio
oscillator
at a frequency of a few Hertz. A larger version can be made with
ping-pong
balls in a large plexiglas cylinder with a motor-driven piston. For a
large
audience, the apparatus is placed on an overhead projector, shadowed
with
an arc lamp against a screen or viewed with a television camera and
monitor.
The amplitude or frequency of the driving oscillation can be changed to
illustrate the effect of changing the temperature of a gas. A lid
resting
on the balls but free to move upward illustrates the pressure exerted
by
molecules in motion as they bombard the walls of their container. One
should
emphasize that this demonstration is merely a simulation since typical
molecules in a gas are about ten million times smaller than the balls
used
in the demonstration.
DISCUSSION
Simulations such as this provide a useful means for visualizing the
motion
of molecules[1]. An ideal gas obeys the ideal gas law
pV = NkT
where p is the pressure, V is the volume, N is the number of
molecules
in the volume, k is Boltzmann's constant (1.38 x 10-23 J/K)
and T is the temperature (in Kelvin). Thus with a given number of
molecules
(N) in a constant volume (V) the pressure increases in proportion to
the
temperature. If the volume (V) is allowed to change but the pressure
(p)
is kept constant by having a movable lid of given weight, the volume
(V)
increases in proportional to the temperature. This is known as
Charles's
Law after Jacques Alexandre Cesar Charles (1746-1823).
Real gases would typically be in thermal equilibrium with the walls
of their container, and thus the molecules would on average gain as
much
energy as they lose upon collision with the walls and with one another.
In this case, the kinetic energy of the balls is considerably greater
than
their thermal energy at room temperature (about kT) and thus the balls
lose energy every time they collide. Hence it is necessary to provide
energy
input to keep the balls moving. The effective "temperature" of the
simulated
gas can be calculated from
kT = mv2
where m is the mass of the balls and v is their velocity. If the
balls
are 0.01 kg and their velocity is 10 m/s, the temperature is about 1023
degrees!
This demonstration also illustrates how apparent randomness can
arise
from simple equations of motion if there are many particles
interacting.
Even without gravity and with perfectly elastic collisions (no energy
loss),
the motion is very complicated and effectively unpredictable after many
collisions. If one of the balls were painted red and its motion
followed
through many collisions, it would execute a random walk (Brownian
motion).
HAZARDS
Take care not to burn out the speaker with too large of an input
voltage.
A handful of ball bearings carelessly spilled on the floor can make for
treacherous walking!
REFERENCE
1. B. Z. Shakhashiri, Chemical Demonstrations, The University
of
Wisconsin Press: Madison, Wisconsin, Vol 2 (1985).
2.21
Firehose Instability
A rubber hose connected to a source of compressed air dangles from the
ceiling and flails about in a complicated fashion.
MATERIALS
-
rubber hose
-
source of compressed air
PROCEDURE
A rubber hose is suspended from the ceiling with one end open and just
above head level. The other end is connected to a source of compressed
air. The audience is asked what would happen if they were to turn on a
garden hose full blast without holding on to the end of it. Most people
answer by flailing their arms wildly. Point out that this effect is
called
the "firehose instability," and that it can be very dangerous with a
large
hose such as used by firefighters. Then turn on the compressed air to
illustrate
that the same thing happens with gases as with liquids. Point out that
it is the same compressed air that one would use to inflate an
automobile
tire or bicycle tire. Try hoses of different diameters and with
different
nozzles to get the best effect for the length and pressure used. A
similar
phenomenon is observed when a balloon inflated with air is released.
DISCUSSION
A hose with fluid coming out one end is unstable in the same way a
pencil
balanced on its point is unstable. If the hose were perfectly straight,
the reaction force of the exhausted fluid would be along the axis of
the
hose and would simply compress it without any sideward motion. With a
stiff
hose or pipe, this is just what would happen. This condition is one of
unstable equilibrium just as a pencil balanced vertically on its point
has all the force along its axis. However, if the hose bends slightly,
there is a horizontal component of the force that causes it to bend
more,
and the bend continues to grow. In the same way, if the pencil is not
exactly
vertical, there is a component of the force that causes it to continue
moving away from the vertical.
The instability actually explains only the initial motion.
Thereafter
there is a complicated interplay of the reaction force, gravity and
elastic
forces in the hose that result in chaotic motion. In principle, it
would
be possible to calculate the resulting motion, and with a computer, it
would even be practical, but the prediction would be precise only for
short
times because of the extreme sensitivity to the initial conditions and
to the exact nature of the forces. Like all chaotic processes, this
demonstration
illustrates apparently random behavior of deterministic systems.
HAZARDS
Hanging the hose above head level prevents it from hitting anyone in
the
face. Be sure it is suspended securely. Scaled-up versions of this
demonstration
using high pressure gases and liquids and large hoses are impressive
but
entail considerable obvious dangers.
2.22
Dripping Faucet
A dripping faucet illustrates periodic and chaotic behavior and the
period
doubling route to chaos.
MATERIALS
-
water faucet or valve with fine control
-
source of water
-
aluminum pie pan
-
microphone, amplifier and speaker (optional)
-
oscilloscope (optional)
PROCEDURE
Place an aluminum pie pan, bottom-side up, about half a meter below a
water
faucet whose drip rate can be controlled. The pie pan makes the
dripping
water audible. A microphone placed under the pie pan and connected to
the
an amplifier and speaker makes an impressive sound easily heard by a
large
audience. The output of the microphone can also be connected to an
oscilloscope
adjusted to a slow sweep rate (about 1 Hz) and set to trigger on the
input
sound. A television camera aimed at the point where the drops are
released
improves visibility.
At a low drip rate (once per second, say) the dripping is perfectly
periodic. As the drip rate is slowly increased, there comes a point
where
a bifurcation occurs and the dripping suddenly changes to a period
twice
as long. The bifurcation is evident in the sound, which should change
from
"drip - drip - drip" to "drip drip - drip drip - drip drip." Careful
adjustment
and patience is required to detect the bifurcation. At a slightly
larger
drip rate, a second bifurcation occurs (four times the period), and so
forth, but these longer periods are difficult to observe. At the end of
this infinite, period-doubling sequence, the dripping is chaotic, and
has
no detectable pattern. It should be easy to hear and observe on an
oscilloscope
the chaotic condition.
DISCUSSION
The dripping faucet is one of the simplest and most common examples of
the period-doubling route to chaos. It has been extensively
studied[1-5],
and an entire scientific book[6] has been written about it. Its
simplicity
makes it ideal for home experimentation.
The mechanism is as follows. There is a maximum size that a drop can
have for its weight to be less than the surface tension that holds it
together
and holds it attached to the rim of the faucet. When it reaches this
critical
size, the drop splits into two parts, one of which falls while the
other
is left behind. The drop left behind then begins to grow, and the
process
repeats. If the drop fills with water at a constant rate, the time
required
for it to reach this critical size is a constant, and the drops are
released
periodically. However, the drop left behind will oscillate up and down
a few times before coming to rest, and inertia of this moving mass will
either encourage or discourage the next release of a drop depending on
the phase of the oscillation when the drop reaches its critical size. A
drop released early will leave more water behind for the formation of
the
next drop and vice versa. It is reasonable that there might be a
condition
in which alternate drops are in and out of phase with the oscillation,
respectively, and this is the situation that leads to period doubling.
Chaos occurs when the drip rate is faster than the oscillation
frequency
so that the phase of the oscillation is different each time a drop is
released.
The oscillation can be easily seen if the faucet is observed closely.
Water drops are a useful analogy to nuclear processes. There is a
maximum
size of the nucleus determined by a competition of the electrostatic
repulsion
between the protons and the short-range attractive nuclear force that
behaves
somewhat like surface tension. When a nucleus exceeds a certain
critical
size, it splits by the fission process with a large release of energy.
The fission fragments are of varying size, probably determined by the
phase
of oscillations within the nucleus when the fission occurs.
HAZARDS
The only hazards are from spilled water and perhaps water damage to the
microphone.
REFERENCES
1. P. Martien, S. C. Pope, P. L. Scott and R. S. Shaw, Phys. Lett 110Z,
399 (1985).
2. X. Wu, E. Tekle and Z. A. Schelly, Rev. Sci. Instr. 60,
3779
(1989).
3. X. Wu and Z. A. Schelly, Physica D 40, 433 (1989).
4. H. Yepez, N. Nuniez, A. L. Salas Brito, C. A. Vargas and L. A.
Vicente,
Eur. J. Phys. 10, 99 (1989).
5. R. F. Calalan, H. Leidecker and G. D. Cahalan, Comp. in Phys. 4,
368 (1990).
6. R. Shaw, The Dripping Faucet as a Model Chaotic System,
Ariel
Press: Santa Cruz, CA (1984).
[Next Chapter] [Previous
Chapter] [Introduction] [Server
Home]
J. C. Sprott
 The
pipe is
supported by a ring stand and connected to a source of compressed gas.
A gas lighter than air, such as helium,* allows the bubbles
to rise to the ceiling as they are released from the pipe. The gas
should
be allowed to flow through the pipe briefly before filling it with soap
solution in order to expel the air. If the bubbles are blown with
hydrogen,
they can be ignited with a candle held in the hand as they rise[2,3].
Some
oxygen can be mixed with the hydrogen, using the arrangement shown in
the
figure, to make a much louder explosion. The loudest explosion will
occur
when the hydrogen-to-oxygen ratio has a stoichiometric value of 2:1 so
as to produce pure H2O. Natural gas, often conveniently
available
on the lecture bench, makes a very beautiful and quiet flame. Natural
gas
consists primarily of methane (CH4) which is slightly less
dense
than air. Mixtures of methane and oxygen will rise only if the ratio of
CH4 to O2 is large. The effect is best if viewed
against a dark background in subdued light.
The
pipe is
supported by a ring stand and connected to a source of compressed gas.
A gas lighter than air, such as helium,* allows the bubbles
to rise to the ceiling as they are released from the pipe. The gas
should
be allowed to flow through the pipe briefly before filling it with soap
solution in order to expel the air. If the bubbles are blown with
hydrogen,
they can be ignited with a candle held in the hand as they rise[2,3].
Some
oxygen can be mixed with the hydrogen, using the arrangement shown in
the
figure, to make a much louder explosion. The loudest explosion will
occur
when the hydrogen-to-oxygen ratio has a stoichiometric value of 2:1 so
as to produce pure H2O. Natural gas, often conveniently
available
on the lecture bench, makes a very beautiful and quiet flame. Natural
gas
consists primarily of methane (CH4) which is slightly less
dense
than air. Mixtures of methane and oxygen will rise only if the ratio of
CH4 to O2 is large. The effect is best if viewed
against a dark background in subdued light.
 Although heat at first appears to have nothing to do with motion, it is
now understood that heat is the motion of molecules. The connection
between
heat and motion was provided by Benjamin Thompson (1753-1814), an
American
who sympathized with the British during the Revolutionary War and
eventually
settled in Bavaria and became Count Rumford[1]. While watching the
boring
of artillery cannons in Munich in 1798, he concluded that motion could
be transformed into heat and estimated the mechanical equivalent of
heat.*
Half a century later the experiment was refined and repeated by James
Prescott
Joule (1818-1889), the son of an English brewer. Joule's careful
quantitative
measurements became a model for modern science[2]. For our purposes, we
will include in the chapter on heat such topics as fluid mechanics,
phase
transitions, cryogenics, thermodynamics, kinetic theory and combustion.
Although heat at first appears to have nothing to do with motion, it is
now understood that heat is the motion of molecules. The connection
between
heat and motion was provided by Benjamin Thompson (1753-1814), an
American
who sympathized with the British during the Revolutionary War and
eventually
settled in Bavaria and became Count Rumford[1]. While watching the
boring
of artillery cannons in Munich in 1798, he concluded that motion could
be transformed into heat and estimated the mechanical equivalent of
heat.*
Half a century later the experiment was refined and repeated by James
Prescott
Joule (1818-1889), the son of an English brewer. Joule's careful
quantitative
measurements became a model for modern science[2]. For our purposes, we
will include in the chapter on heat such topics as fluid mechanics,
phase
transitions, cryogenics, thermodynamics, kinetic theory and combustion.
 Two to
three
milliliters of >95% ethanol (C2H5OH) is placed
in
a 250-milliliter polyethylene bottle with two nails or screws through
the
sides as shown in the illustration[1]. The tips of the nails or screws
are pointed and come within a few millimeters of touching. The bottle
is
sealed with a cork or rubber stopper. The bottle can be shaken briefly
to ensure that the liquid ethanol has come to equilibrium with its
vapor.
The nails are then connected to a high-voltage (>1000 volts),
low-current
source. The resulting spark ignites the ethanol and blows the stopper
off.
Alternately, a hand-held Tesla coil can be held near one of the nails.
Even with the second nail disconnected, there is enough capacitance
between
the nails to cause a spark to jump across the gap. The capacitance can
be increased, causing a more intense spark, by connecting the second
nail
to ground or to any conductor with a large surface area. A number of
such
bottles can be placed in series electrically, causing all the stoppers
to blow off at the same time. Larger bottles can be used for more
impressive
explosions. One can point out that cars can use ethanol as fuel and
that
the reaction in the cylinders of the engine is the same as the one
illustrated
here.
Two to
three
milliliters of >95% ethanol (C2H5OH) is placed
in
a 250-milliliter polyethylene bottle with two nails or screws through
the
sides as shown in the illustration[1]. The tips of the nails or screws
are pointed and come within a few millimeters of touching. The bottle
is
sealed with a cork or rubber stopper. The bottle can be shaken briefly
to ensure that the liquid ethanol has come to equilibrium with its
vapor.
The nails are then connected to a high-voltage (>1000 volts),
low-current
source. The resulting spark ignites the ethanol and blows the stopper
off.
Alternately, a hand-held Tesla coil can be held near one of the nails.
Even with the second nail disconnected, there is enough capacitance
between
the nails to cause a spark to jump across the gap. The capacitance can
be increased, causing a more intense spark, by connecting the second
nail
to ground or to any conductor with a large surface area. A number of
such
bottles can be placed in series electrically, causing all the stoppers
to blow off at the same time. Larger bottles can be used for more
impressive
explosions. One can point out that cars can use ethanol as fuel and
that
the reaction in the cylinders of the engine is the same as the one
illustrated
here.